Learn about Cannabidiol CBD
Cannabidiol CBD is arguably one of the most important medical innovations of the twenty-first century. It has been used safely for thousands of years, but we are only just now beginning to unlock its true potential. It's no exaggeration to say that the breakthroughs that change how we understand CBD are coming on a near-daily basis. From medicine to skincare to personal care and beyond, CBD is poised to positively impact many aspects of our lives for coming years.

"The growing body of science and research supporting CBD as a valid medicine with an extraordinary range of potential applications can simply no longer be ignored."
- Leonard Leinow in CBD: A Patient's Guide to Medical Cannabis
Cannabinoids Knowledgebase

CBD is the second most common active compound within the marijuana plant. It can be derived in relatively large quantities from hemp to be used for medical treatment. CBD has many potential applications for sufferers of chronic disease. CBD has rapidly become a popular area of medical study for several reasons. First, it does not produce a high in patients who use it. Second, it does not appear to cause any symptoms of chemical dependency. The low potential for abuse or addiction makes it highly promising. Promising CBD medical discoveries point to the possibility that it might be useful for a wide range of conditions throughout life. The more doctors come to understand how CBD affects the perception of pain, the easier it will be to prescribe effective and safe doses.
CBD reacts with cannabinoid receptors throughout the human body. CBD works to relieve inflammation and pain while producing a calming effect in patients. For this reason, it is often used to treat anxiety and sleep disorders. It has also been shown to work with THC to reduce the size of tumors in cancer patients.
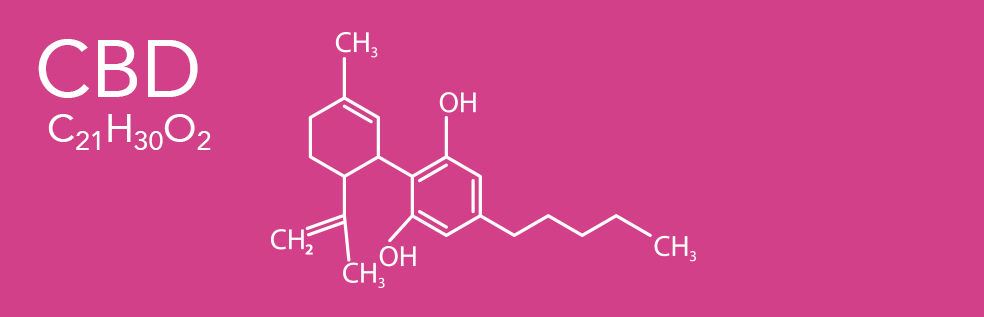
CBD, CBC, and THC share the same molecular formula, C21H30O2, containing twenty-one atoms of carbon, thirty hydrogens, and two oxygen. However their effects are quite different from one another. Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of more than 120 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. As of 2019, clinical research on CBD included studies related to anxiety, cognition, movement disorders, and pain, but there is insufficient high-quality evidence that cannabidiol is effective for these conditions.

Current CBD Clinical Research
CBD inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses
by Long Chi Nguyen , Yang , Nicolaescu , J. Best , Gula , Divyasha, Saxena, Jon D. Gabbard, Shao-Nong , Ohtsuki, Marsha Rich Rosner | January 20, 2022 | Science Advances | Study Details
ABSTRACT
The spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and ongoing coronavirus disease 2019 (COVID-19) pandemic underscores the need for new treatments. Here, we report that cannabidiol (CBD) inhibits infection of SARS-CoV-2 in cells and mice. CBD and its metabolite 7-OH-CBD, but not THC or other congeneric cannabinoids tested, potently block SARS-CoV-2 replication in lung epithelial cells. CBD acts after viral entry, inhibiting viral gene expression and reversing many effects of SARS-CoV-2 on host gene transcription. CBD inhibits SARS-CoV-2 replication in part by up-regulating the host IRE1α ribonuclease endoplasmic reticulum (ER) stress response and interferon signaling pathways. In matched groups of human patients from the National COVID Cohort Collaborative, CBD (100 mg/ml oral solution per medical records) had a significant negative association with positive SARS-CoV-2 tests. This study highlights CBD as a potential preventative agent for early-stage SARS-CoV-2 infection and merits future clinical trials. We caution against current use of non-medical formulations as a preventative or treatment therapy.

Assessing the Effects of a CBD From Hemp Supplement in Healthy Adults
by Shawn M. Arent, University of South Carolina | January 28, 2022 | ClinicalTrials.gov | Study Details
ABSTRACT
Cannabis contains several phyto-cannabinoids among which Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are most widely known. THC is the main compound responsible for the psychoactive properties and also deemed responsible for several side-effects associated with cannabis. CBD, on the other hand, is not a strong cannabinoid receptor agonist and lacks psychotropic activity. However, due to its affinity for several other target sites, it is being studied for potential pharmacological properties. The diverse range of interactions at different receptor sites in the human body is believed to be responsible for therapeutic efficacy of CBD in treating kidney fibrosis, metabolic syndrome, anorexia, obesity, amelioration of osteoarthritis as well as several other musculoskeletal diseases. Recent research has also explored the use of CBD to relieve stress and depression, likely due to its agonistic influence on the 5-HT3 receptors as well as improving hippocampal neural growth and development. CBD has also been studied for its anti-oxidant activity, deemed on-par to that of Vitamin C in laboratory studies. The effect of CBD on inflammation and the immune system has been studied. The sedative effects of CBD have been investigated for the potential use of CBD as an anxiolytic and to improve mood as well as sleep. Recent studies have also explored the analgesic and pain-relieving properties of CBD, making it a suitable candidate that needs further investigations. Interestingly, a recent systematic review explored the use of CBD in viral diseases, with several pre-clinical studies indicating CBD as an effective candidate against viral disease.
With the spread of the coronavirus disease (COVID-19) pandemic, there has been a strong interest in developing therapies to eliminate or reduce the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. SARS-CoV-2 uses Angiotensin Converting Enzyme (ACE) receptors to gain entry into the human body and penetrate the respiratory system. In a recent in vitro study, pretreatment with CBD soap in cells expressing ACE-2 receptor was effective in inhibiting the replication of SARS-CoV-2 in those cells. This is an interesting finding where further research is needed to study the influence of CBD consumption on ACE activity. Several studies of CBD safety have demonstrated lack of any safety concerns over a range of different doses. A recent safety study of CBD by Bergamaschi et al. demonstrated absence of any influence on the central nervous system, vital signs or mood changes as well as lack of any side effect observed for doses up to 1500 mg/day (orally) or 30 mg/day (intravenously).It has been hypothesized that the trace amounts of THC present within the CBD extract could potentially be responsible for any side-effects. Therefore, CBD is considered very safe for human consumption in the dose being tested in this study (less than 200mg/day). Additional clinical research is required to confirm and support therapeutic use of CBD for being effective in modulating ACE expression, mood, stress, anti-inflammatory, antioxidant, immunomodulating, sedative & anxiolytic, analgesic, pain relieving and anti-viral therapeutic claims. This research will also help to understand any safety issues with the long-term regular use of CBD COA on healthy adults. Therefore, this prospective, randomized, double-blind, placebo controlled study will be conducted to explore the physiologic, biochemical, and psychometric impacts of a brand-specific hemp-derived CBD product in healthy adults.

Evaluation of the Interactions of Cannabidiol CBD With Morphine
National Institute on Drug Abuse (NIDA) | December 3, 2021 | ClinicalTrials.gov | Study Details
ABSTRACT
This is an inpatient, single-blind, non-randomized, 1-sequence study involving healthy subjects who have used opioids for recreational use. The primary objective of the study is to establish the pharmacokinetic parameters of morphine 30 mg when administered with and without CBD 350 mg b.i.d. and CBD 700 mg b.i.d.

Effect of Medical Cannabis for Non-motor Symptoms of Parkinson's Disease
Dr. Saar Anis, Sheba Medical Center | November 3, 2021 | ClinicalTrials.gov | Study Details
ABSTRACT
Medical cannabis (MC) is a standard treatment in Israel to Parkinson's disease (PD) patients suffering from pain. Nevertheless, it is not known about MC effectiveness for other non-motor symptoms of the disease. Our aim is to prospectively observe patients with PD before and after initiation of MC, for non-motor symptoms effect. In specific, relying of data from multiple sclerosis patients and basic science showing cannabinoid receptor 1 (CB1) is abundantly expressed in the sub epithelial layer of the bladder, we will explore the impact of MC on bladder function and urinary symptoms. This is a prospective, open-label, observational study. Patients with Parkinson's disease (PD) receiving licensure from Israeli Ministry of Health (MOH) for using medical cannabis (MC) for PD related symptoms and pain, being followed up in the Movement Disorders Institute (MDI) at SHEBA Medical Center (SMC) will be eligible to participate. Assessment regarding patient demographic, disease characteristics (Hoehn and Yahr, disease duration, disease first symptom etc.) will be collected at baseline along with designated questionnaires to evaluate the non-motor symptoms (NMSS, PDSS, Kings PD pain scale, PDQ8) and urinary function (Bladder over activity, International prostate symptom score (IPSS) and nocturia questionnaires). after MC initiation, patients will be observed and evaluated for the impact of MC 4-8 weeks following treatment initiation. Also, for each patient, MC being used will be analyzed in order to expose relationship between phyto-cannabinoid content and efficacy or side effects.

Cannabidiol for Reduction of Brain Neuroinflammation CBD
Jodi Gilman, Massachusetts General Hospital | October 4, 2021 | ClinicalTrials.gov | Study Details
ABSTRACT
This study will investigate whether cannabidiol (CBD), the primary centrally and peripherally active non-intoxicating compound in the cannabis plant, exerts anti-neuroinflammatory effects in patients with chronic low back pain (cLBP) with or without mild-to-moderate depression. This is a randomized, double-blind, 2-arm mechanistic trial that seeks to assess the effects of CBD and placebo in patients with cLBP with and without mild-to-moderate depression, using integrated positron emission tomography / magnetic resonance imaging (PET/MRI) scans. The use of integrated PET/MRI will make it possible to simultaneously evaluate neuroinflammation (using [11C]PBR28, a second-generation radioligand for TSPO) and striatal function (using the Monetary Incentive Delay task, a validated fMRI task that probes behavioral and neural responses to rewards and losses).

Antioxidative and Anti-Inflammatory Properties of CBD
by Sinemyiz Atalay, Iwona Jarocka-Karpowicz and Elzbieta Skrzydlewska | December 25, 2019 | Antioxidants 2020 |
ABSTRACT
Cannabidiol (CBD) is one of the main pharmacologically active phytocannabinoids of Cannabis sativa L. CBD is non-psychoactive but exerts a number of beneficial pharmacological effects, including anti-inflammatory and antioxidant properties. The chemistry and pharmacology of CBD, as well as various molecular targets, including cannabinoid receptors and other components of the endocannabinoid system with which it interacts, have been extensively studied. In addition, preclinical and clinical studies have contributed to our understanding of the therapeutic potential of CBD for many diseases, including diseases associated with oxidative stress. Here, we review the main biological effects of CBD, and its synthetic derivatives, focusing on the cellular, antioxidant, and anti-inflammatory properties of CBD.

Neurological Aspects of Medical Use of CBD
by Carmen Mannucci, Michele Navarra, Fabrizio Calapai1, Elvira Ventura Spagnolo,Francesco Paolo Busardò, Roberto Da Cas, Francesca Menniti Ippolito and Gioacchino Calapai1 | March 8, 2007 | CNS & Neurological Disorders - Drug Targets, 2017 | Full Text Article
ABSTRACT
Cannabidiol (CBD) is one of the main pharmacologically active phytocannabinoids of Cannabis sativa L. CBD is non-psychoactive but exerts a number of beneficial pharmacological effects, including anti-inflammatory and antioxidant properties. The chemistry and pharmacology of CBD, as well as various molecular targets, including cannabinoid receptors and other components of the endocannabinoid system with which it interacts, have been extensively studied. In addition, preclinical and clinical studies have contributed to our understanding of the therapeutic potential of CBD for many diseases, including diseases associated with oxidative stress. Here, we review the main biological effects of CBD, and its synthetic derivatives, focusing on the cellular, antioxidant, and anti-inflammatory properties of CBD.

Clinical Trials of CBD for Substance Use Disorders: Outcome Measures, Surrogate Endpoints, and Biomarkers
Alix Morel1, Pierre Lebard1, Alexandra Dereux, Julien Azuar, Frank Questel, Frank Bellivier, Cynthia Marie-Claire, Mélina Fatséas, Florence Vorspan, Vanessa Bloch | February 22, 2021 | Frontiers in Psychiatry | Full Text Article
ABSTRACT
Cannabidiol (CBD) is a cannabinoid of potential interest for the treatment of substance use disorders. Our aim was to review the outcome measures, surrogate endpoints, and biomarkers in published and ongoing randomized clinical trials. We conducted a search in PubMed, Web of Science, PMC, PsycINFO, EMBASE, CENTRAL Cochrane Library, “clinicalTrials.gov,” “clinicaltrialsregister.eu,” and “anzctr.org.au” for published and ongoing studies. Inclusion criteria were randomized clinical trials (RCTs) examining the use of CBD alone or in association with other cannabinoids, in all substance use disorders. The included studies were analyzed in detail and their qualities assessed by a standardized tool (CONSORT 2010). A short description of excluded studies, consisting in controlled short-term or single administration in non-treatment-seeking drug users, is provided. The screening retrieved 207 published studies, including only 3 RCTs in cannabis use disorder. Furthermore, 12 excluded studies in cannabis, tobacco, and opioid use disorders are described.
Primary outcomes were validated withdrawal symptoms scales and drug use reduction in the three RCTs. In the short-term or crossover studies, the outcome measures were visual analog scales for subjective states; self-rated scales for withdrawal, craving, anxiety, or psychotomimetic symptoms; and laboratory tasks of drug-induced craving, effort expenditure, attentional bias for substance, impulsivity, or anxiety to serve as surrogate endpoints for treatment efficacy. Of note, ongoing studies are now adding peripheral biomarkers of the endocannabinoid system status to predict treatment response. The outcome measures and biomarkers assessed in the ongoing CBD trials for substance use disorders are improving.


