GMP Program Compliance

Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to rigid quality standards. It is designed to minimize the risks involved in any CBD product manufacturing production that cannot be eliminated through testing the final product.
GMP covers all aspects of production from the starting materials, premises, and equipment to the training and personal hygiene of staff. Detailed written procedures and consistent record-keeping are essential for each process that could affect the quality of the finished product. There must be systems to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process - every time a product is made.
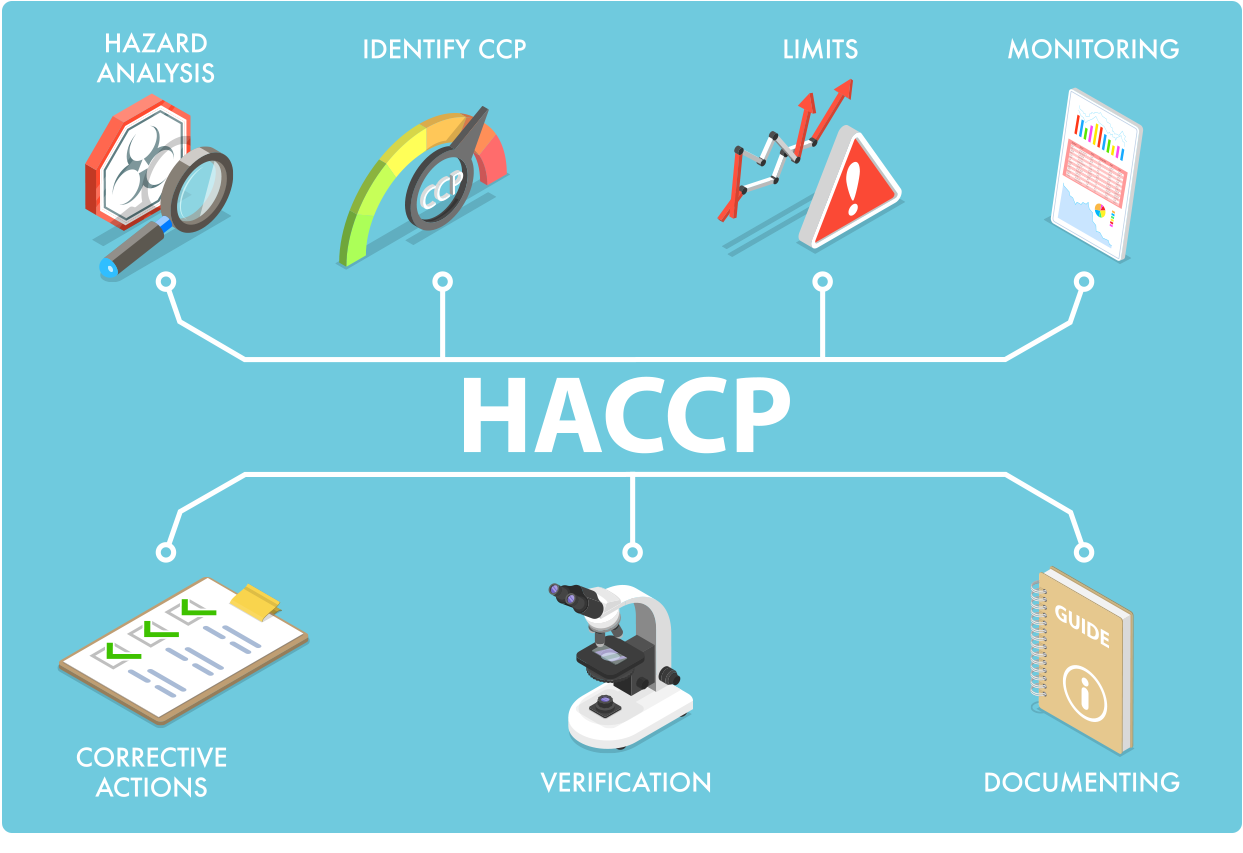
Our Hazard Analysis and Critical Control Points Plan
Our HACCP (Hazard Analysis and Critical Control Points) Plan applies to the product manufacturing safety management system, uses the approach of controlling critical control points (CCPs) in ingestible and topical product processing and packaging to prevent potential safety hazards. The system is science-based and systematic, which identifies biological, chemical and physical hazards and measures for their control to ensure the safety of finished products. HACCP is based on prevention and reduction of the reliance on product inspection and testing. This HACCP Plan is capable of accommodating changes, such as formula, recipe or equipment changes, processing procedures or technological developments.
Management commitment is necessary for an effective implementation of the HACCP System. The Covalent Custom Cannabinoids management and HACCP team members are strongly committed to implementing, monitoring and verifying of the HACCP plan by providing adequate training, monitoring, and safety systems documentation and testing. The Covalent HACCP team in collaboration with the safety systems consultants have developed the current HACCP plan accordingly.
Our Personnel Practices Program
Proper hygiene greatly reduces the potential for product contamination. Contaminated food products can transmit foodborne illness. 20-50% of adults carry pathonogenic bacteria in their noses. 20-35% of adults can carry pathogenic bacteria on their skin. Personal hygience practices for all personnel of Covalent CC, LLC are a Critical Control Point (CCP) in our 360° Quality Assurance Systems. A high standard of personal cleanliness is required for all personnel in our facility. Proper hygiene can prevent contamination of ingredients, products or packaging. All employees must follow the rules for working in our manufacturing and production facility.

100% Traceability Ensured
We designed the Enterprise Resource Planning data system that we use every moment in a work day to require lot coding and batch tracking for every ingredient and material used in our production processes. Ever milligram of cannabinoid concentrate is traceable, as is every milliliter of carrier oil, flavor agent, every bottle, label, safety seal and carton. Fortunately we have not encountered an adverse event that required a product recall to-date and we believe that is very much by design by working with intention to do everything the right way in an industry that is currently self-regulating.

Rigorous laboratory testing ensures the quality and integrity of our products.
Covalent Custom Cannabinoids has set the industry standard in hemp cannabinoid testing and quality control. We require a 3rd party test from our extraction labs upon completion of each and every production batch. Upon receipt of raw materials, we immediately send samples to our own trusted third party labs to confirm cannabinoid potency, as well as the safety of the material to ensure that it is free of microbials, heavy metals, residual solvents and pesticides. Only then do we release raw materials for distribution or manufacturing finished products. This is the way.

Partner with Covalent
Register My B2B Wholesale Account
Purchase Online
- Broad Spectrum Distillate
- Crystal-Resistant Distillate
- Full Spectrum Distillate
- Full Spectrum Whole Hemp Extract
- Cannabidiol (CBD) Isolate
- Cannabidiolic Acid (CBDa) Isolate
- Cannabigerol (CBG) Isolate
- Cannabigerolic Acid (CBGa) Isolate
- Cannabinol (CBN) Isolate
- Cannabichromene (CBC) Isolate
- THC-Free Water Soluble Serum
- THC-Free Water Soluble Liquid
- THC-Free Water Soluble Powder
- Full Spectrum Water Soluble Liquid
- Full Spectrum Water Soluble Powder
- Custom CBD Tinctures
- Cannabinoid Infused CBD Gummies
- Cannabinoid Infused CBD Softgels
- Cannabinoid Infused CBD Honey Sticks
- Pain Relief CBD Topicals
- Bath & Body CBD Topicals
- Skincare & Beauty CBD Topicals
- Custom CBD Topicals
- Pet Health & Wellness CBD Products
- Retail-Ready CBD Products



